Luminex® multiplex technology: the benefits
- Fast: 20 minutes sample incubation, 20 minutes conjugate incubation. Analysis result in less than 3 hours
- High-throughput assay: ideal test for high sample throughput
- Automation with Dynablot: fully automated processing and evaluation possible. Connection to the laboratory information system (LIS) possible
- Precise: very high measurement accuracy and reproducibility of the test results
- All in one: the detection of antibodies against individual antigens combines the advantages of ELISA and confirmatory tests
- Low sample volume: 10 μl is sufficient for serum, plasma and CSF
- Flexible combination: different parameters and conjugate classes on one plate possible with same incubation times
- Safety thanks to integrated controls: incubation control, conjugate control, negative control
Luminex® multiplex technology: test principle
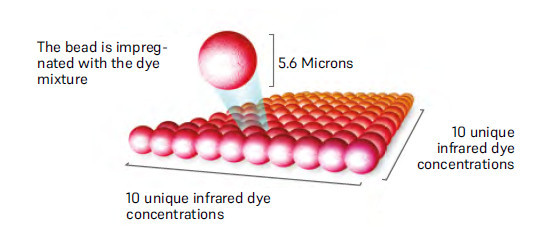
Colour-coded differentiation of up to 100 different bead populations, specific simultaneous analysis of up to 100 parameters in one sample possible. Utilisation of magnetic polystyrene beads (MagPlex®) for the highest recovery rate.
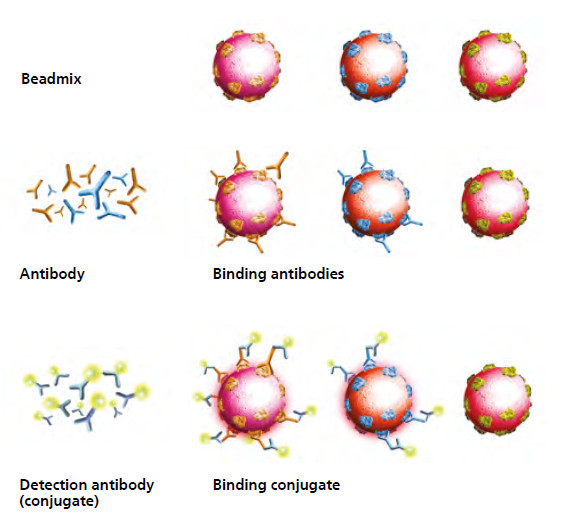
1. Antibody detection with recomBead tests
- Multianalyte assays based on immunodominant, diagnostically relevant antigens.
- Addition of patient serum to the bead mix provided: bound antibodies are labelled with a specific detection antibody (conjugate).
- The antigen-antibody complexes are analysed and the results are clearly displayed on the analyser. The different colouring assigns the beads to their respective population/antigen, the fluorescence intensity results from the amount of bound antibodies.
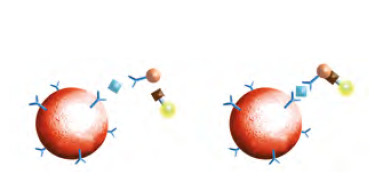
2. CXCL13 Sandwich technology
- Anti-hCXCL13: covalent binding to the magnetic beads. The quantification of the target antigen CXCL13 in the CSF sample is carried out using a sandwich assay with anti-hCXCL13 antibodies and a biotinstreptavidin reporter system.
recomBead portfolio
recomBead Borrelia IgG, IgM 2.0
- Simultaneous but separate detection of individual antigen-specific antibodies – classification into early and late phase of Borrelia infection possible
- Species-specific p18 (Dbpa) antigens of the 5 predominant Borrelia species: B. b. sensu strictu, B. garinii, B. afzelii, B. spielmanii, B. bavariensis – optimal setting for the typing of Borrelia bacteria
- Possibility of quantitative determination of VlsE exists (research use only)
- Serum and cerebrospinal fluid diagnostics with determination of the Borrelia-specific antibody index (AI)
- Small sample volume (10 μl) is sufficient – particularly important in CSF diagnostics
recomBead CXCL13 PART A and PART B
- CXCL13: Activity marker for acute neuroborreliosis and other inflammatory diseases of the central diseases of the central nervous system
- Detection of CXCL13/BLC (BLC = B lymphocyte chemoattractant) in human cerebrospinal fluid
- Quantification of CXCL13 antigens to be detected using integrated standard curves
recomBead EBV IgG, IgM 2.
- Reliable detection of past infections with the Epstein-Barr virus due to very high EBNA-1 specificity – the key antigen in EBV serology
- Reliable detection of acute infections due to the very high sensitivity of early antigens
- Simultaneous, separate detection of all relevant EBV antigens
recomBead Yersinia IgG, IgA 2.0
- Screening or confirmation test using recombinant Yersinia antigens: Yersinia enterocolitica and Yersinia pseudotuberculosis
- Detection of all human pathogenic Yersinia using the Yersinia Outer Proteins (YOPs)
- Differentiation between Y. enterocolitica and Y. pseudotuberculosis infection serologically possible for the first time through the use of new species-specific Yersinia antigens (PsaA, MyfA)
- No cross-reactions to Brucella and other pathogens, and no interference from LPS
recomBead Treponema IgG, IgM 2.0
- Reliable detection using immunodominant, recombinant antigens
- Use of the highly specific, minimally cross-reactive treponema antigens Tp47, Tp17, Tp15 and TmpA as well as Tp257 (Gpd) and Tp453
- Can be used as a confirmatory assay for positive or unclear samples form screening
Product added to cart!